Utility Meaning In Pharma Industry
Installation Qualification is a documented process that verifies that critical pieces of equipment software or instruments that directly impact product quality have been. Requirements for the purity of water used in pharmaceutical manufacturing.

Crypto Glossary Blockchain Definition What Is Blockchain Traceitgreen In 2021 Glossary Beginners Guide Blockchain
Quality utilities are a prerequisite for effective poverty eradication.

Utility meaning in pharma industry. As a result these are treated as products that need to satisfy FDA regulatory requirements and pharma manufacturing standards just like raw materials and other equipment used in the industry. Critical Utilities GMP Compliance How to Be Compliant and Ready to Prove It is to help pharmaceutical organizations achieve and maintain their critical utility systems in a state of control and then be able to efficiently demonstrate their systems Good Manufacturing Practice GMP compliance to regulatory inspectors and auditors. In pharmaceuticals critical utilities like water and HVAC Heating Ventilation and Air Conditioning systems form the backbone of the manufacturing process.
Cleanrooms Critical equipment Sanitisation appropriate Sterilization technologies SIP Autoclave. Within the complex facilities utilities and equipment FEU in a manufacturing plant a line in the sand needs to be drawn between GMP-critical and business-critical. APIs include substances manufactured by processes such as 1 chemical synthesis.
All utilities that could affect product quality should be qualified and appropriately monitored and action should be taken when these utility limits are exceeded. If a company strives for innovation theres no room for lagging behind with their software. In such cases steam from a conventional boiler often called utility or plant steam is.
Configured according to standards set by the manufacturer or. Pharmacy is the art and science of preparing and dispensing drugs for preventing diagnosing or treating diseases or disorders in humans and animals. Facility design is critical.
Pharmacokinetics is the study of metabolic processes relating to the absorption distribution biotransformation and elimination of a drug in humans or animals. The goal of the ISPE Good Practice Guide. Baseline Pharmaceutical Engineering Guides ISPE A series of industry publications developed in partnership with the US Food and Drug Administration FDA.
The IQ is described. SOPs for pharmaceuticals related to Quality Assurance Quality Control Production Maintenance Utility and Human Resource are listed here. Better control of drug manufacturing preventive maintenance of equipment and improved supply chain management.
Typical programs begin with installation qualification IQ. Purity sterility and the risk level audit assessment ASTM2500 FMEA. UTILITY SERVICES AND SERVICE FACILITIES UTILITY SERVICES.
Governments are ultimately responsible for ensuring reliable universal access of. Standard Operating Procedures SOPs is a written procedure for any process or system that is followed during the operation of any system or equipment. This means being very specific about what you want both internally and towards your supplier and making energy efficiency a concrete project goal.
Most important of these are heating ventilation and air conditioning HVAC water including clean steam and compressed gases. Each pharmaceutical but also cosmetics food chemical industrys manufacturing process uses several support system with different functions and generated and distributed with centralized installations. UTILITY SERVICES include 1.
Likewise individual utilities require qualification. While the process for identification has been discussed before the following are the differences between a GMP-critical and business-critical FEU including the respective change management taken from a Jorge Cordero. Utilities water electricity and gas are essential services that play a vital role in economic and social development.
The Definition of Marginal Utility. Pharmaceutical engineering covers all parts of drug manufacturing from designing a new facility to optimizing manufacturing processes and perfecting packaging solutions. Large pharmaceutical companies as a group.
These systems are not necessarily designed and customized for users of a single production facility but. Marginal utility MU is defined as the additional cardinal utility gained from the consumption of one additional unit of a good or service or the additional. SOPs in Editable MS-Word Format.
Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis cure mitigation treatment or prevention of disease or to affect the structure and function of the body of humans or other animals. Similarly to AI and machine learning the internet of things IoT provides the pharmaceutical industry with a selection of new opportunities including. Want more articles like this.
Utility Systems in Pharma. Additionally the parameters which pharmaceutical water quality is measured. Pharma definition is - a pharmaceutical company.
HEATING VENTILATION AND AIR CONDITIONING 2. EQUIPMENT USED IN PHARMACEUTICAL INDUSTRY The definition of air handling unit from ANSIAHRI Standard 430-2009 states that it is A factory-made encased assembly consisting of a fan or fans and other necessary equipment to perform one or more of the functions of circulating cleaning heating cooling humidifying dehumidifying and mixing of. Clean steam is used in the pharmaceutical and healthcare industries in processes where the steam or its condensate can come into contact with a pharmaceutical or medical product and cause contamination.
Quick links Contact us. How to use pharma in a sentence. Equipment utilities systems production rooms infrastructure list Cross-Matrix.
Processes clean utilities and pharmaceutical systems functional analysis. Each volume in the series is a collaborative effort of industry leaders representing a broad cross-section of manufacturers and other industry. This is because steam purity is often defined by the purity of the condensate and this is often referenced to one of the published water purity standards.

Pharmaceutical Industry Factory Worker Sponsored Ad Industry Pharmaceutical Worker Factory Pharmaceutical Industry Factory Worker Pharmaceutical

Nuclear Power Plants In The U S Power Plant Map Nuclear Power Plant

Ad Campaign For Physicians Practice Billing Service Healthcare Marketing Digital Marketing Plan Ads
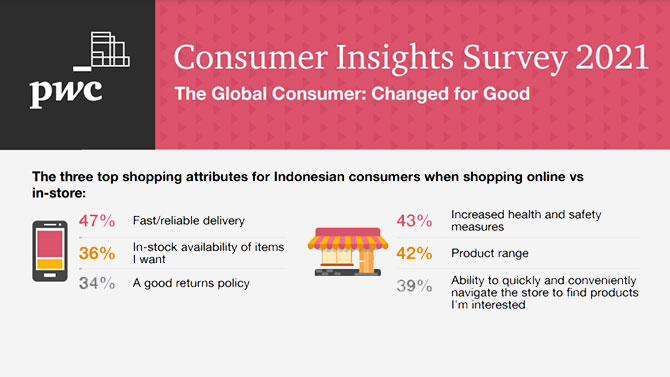
Consumer And Industrial Products And Services

Lumit Ar App Icon By Dem0 Design App Icon Custom Icons Icon
Chapter 79 Pharmaceutical Industry

The 34 Best Interactive Data Visualizations From The New York Times Dolphins Data Visualization Interactive New York Times
Utility System Qualification For The Pharmaceutical Industry Panorama

The V Model Lifecycle For Managing Software Projects Software Projects Integration Testing Development

Industry 4 0 Strategy Consulting Services Bcg
Consumer And Industrial Products And Services

New Digital Health Technology To Help Cancer Patients Wins Top Innovation Prize Digital Health Health Technology Cancer Patients

Image Result For Magazine Technology

Festim Toshi Architectural Standards Utility Architecture Blueprints Architecture Symbols Interior Design Student
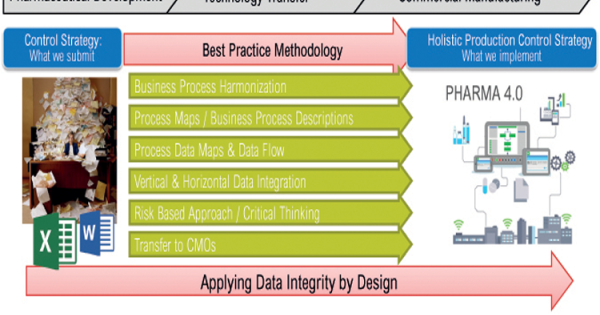
A Holistic Approach To Production Control Pharmaceutical Engineering
Post a Comment for "Utility Meaning In Pharma Industry"